Methanol Ethanol And N-propanol Are Three Common Alcohols. We share all the details with you about alcohols, molecules and other details.
Methanol Ethanol And N-propanol Are Three Common Alcohols
Methanol, ethanol, and n-propanol are three common alcohols. When 1.00 g of each of these alcohols is burned in air, heat is liberated as shown by the following data: (a) methanol (CH3OH), –22.6 kJ/g; (b) ethanol (C2H5OH), –29.7 kJ/g; (c) n-propanol (C3H7OH), –33.4 kJ/g.
Because of the polar hydroxy group and the non-polar alkyl group, alcohols form interesting structure-property relationships that vary with the size of the alkyl group and the number of hydroxy groups. Below, we examine the melting and boiling point, solubility (miscibility), viscosity and flammability in detail in terms of structure-property relations.
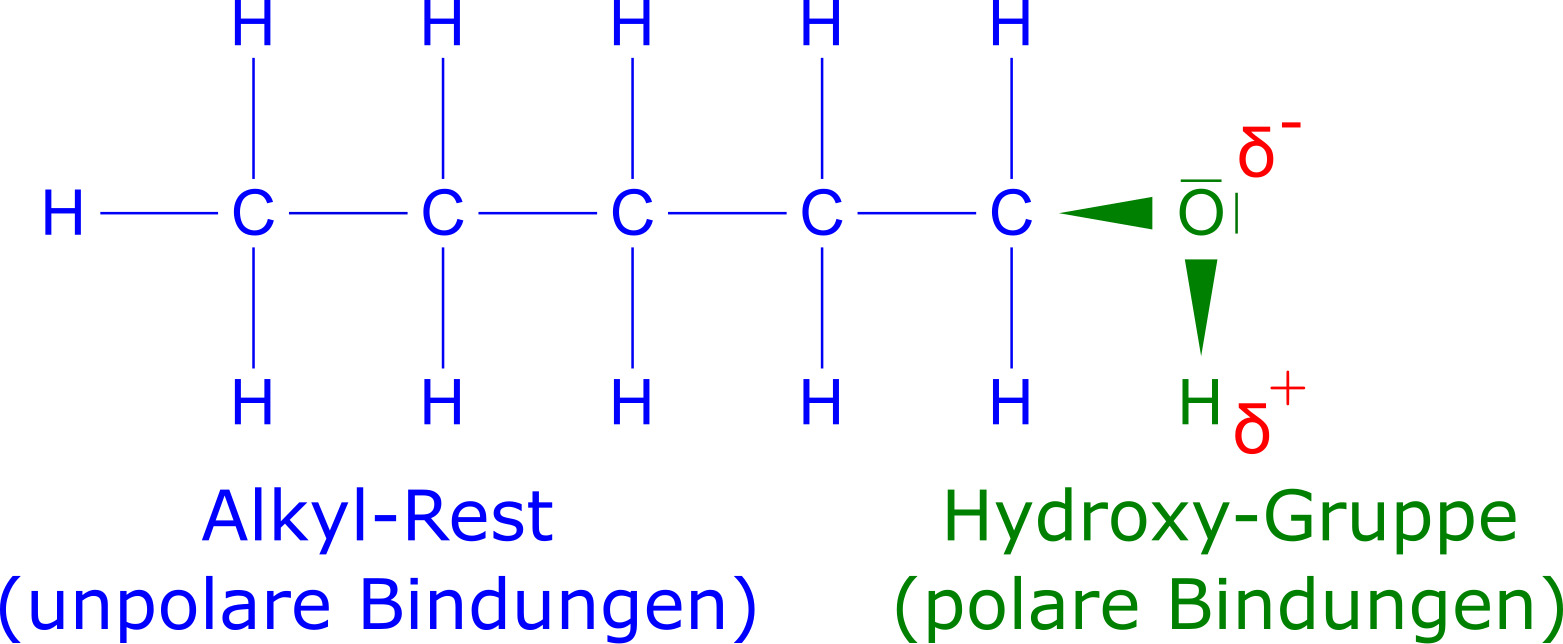
Methanol Ethanol And N-propanol Are Three Common Alcohols: We’ll take a look at the effect of chain length before properties are affected by two factors, then the effect of hydroxy group number. Viscosity refers to the viscosity of liquids and gases (fluids). The more viscous a substance is, the more viscous it is and it is necessary to examine its flammability in terms of structure-property relations.
Melting Point And Boiling Point
Melting at the particle level indicates that the molecules have enough energy to leave their stable regions. The molecules are still dense and disordered. As the energy increases, the speed of the particles also increases. When the particle velocity is high enough to overcome the gravitational forces, the particles are formed separately. Matter evaporates. Since the melting and boiling points depend on the particle velocity, the mass of the molecules and the strength of the attractive forces between the molecules are the most important factors affecting the melting and boiling points.
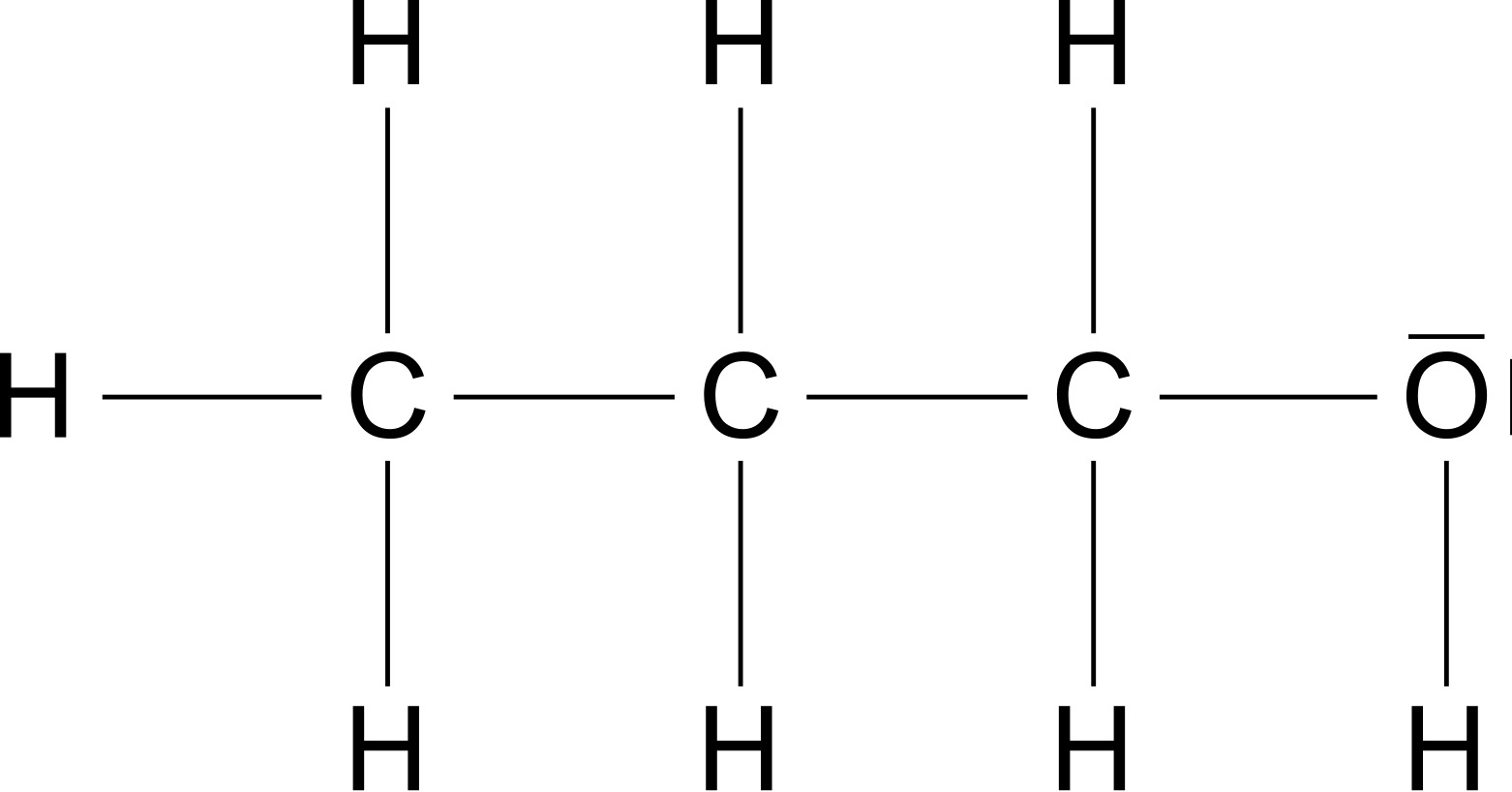
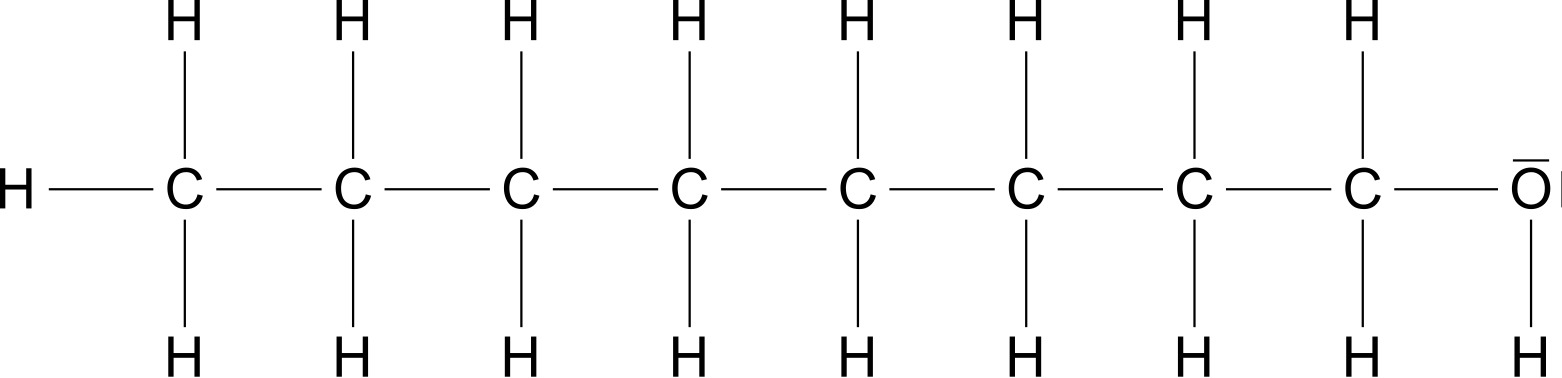
Methanol Ethanol And N-propanol Are Three Common Alcohols – Let’s first compare propanol (Figure 2) and octanol (Figure 3). Both molecules have a hydroxy group and an alkyl radical. Octanol molecule has a higher molar mass \ce{$M$\,(Octanol)\,=\,130,23\frac{g}{mol}} propanol molecule \ce{$M$\,(Propanol)\ ;=\;60,10\frac{g}{mol}}.
Methanol Ethanol And N-propanol Are Three Common Alcohols / Both molecules have a hydroxy group that can form hydrogen bonds. It also has an alkyl residue that can generate van der Waals forces. The difference is, of course, that the surface area of the octanyl residue is greater than that of the propanyl residue. That is, the van der Waals forces between octanyl residues are greater than between propanyl residues. Since both the mass and the attraction force increase as the chain length increases, we see the following general statements: For alkanols with hydroxy groups, the following applies: The longer the chain length of the molecule, the higher the boiling and melting point.
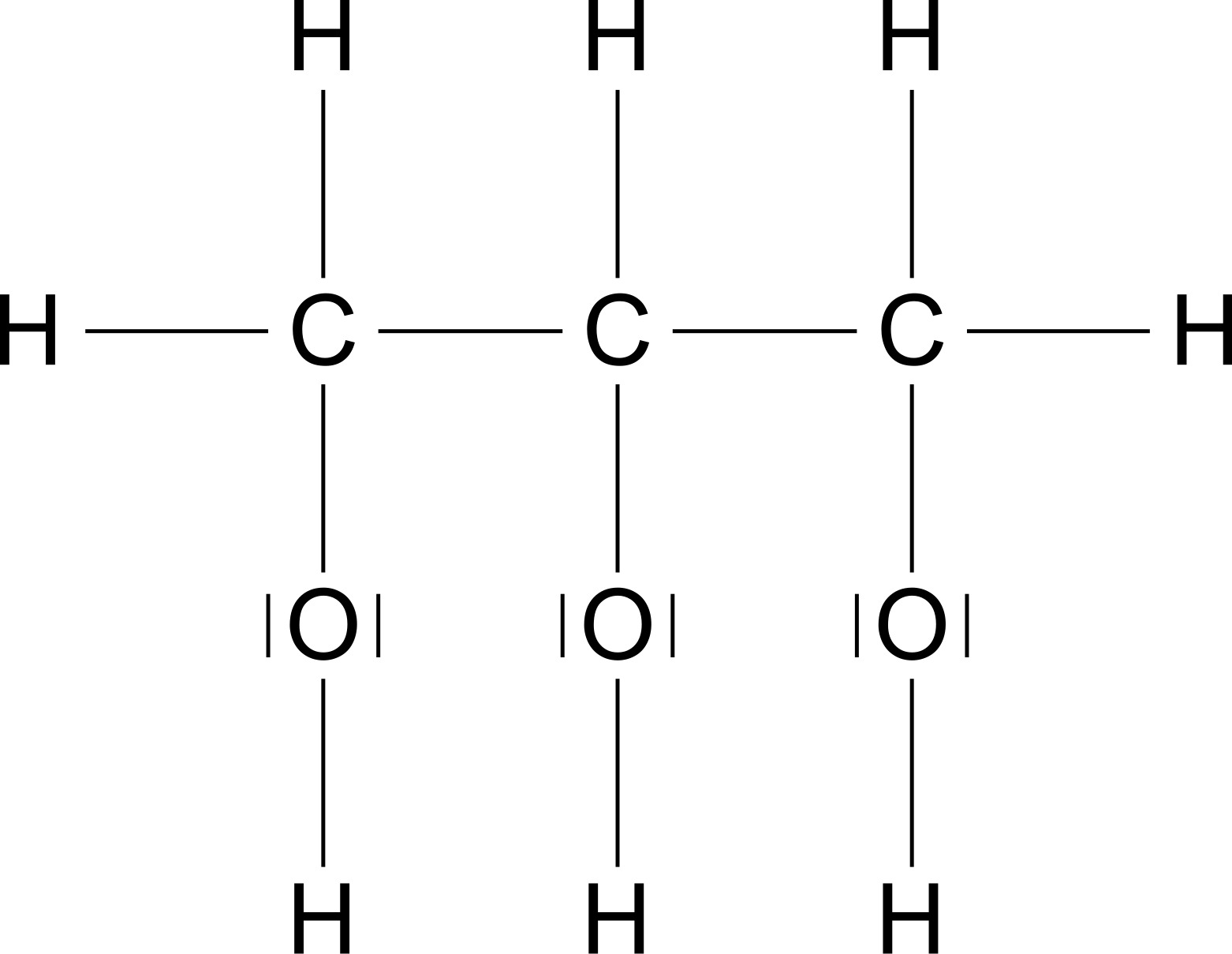
Now let’s compare propanol with glycerol (propane-1,2,3-triol) (Figure 4).
The difference between the two molecules is that each of the other two hydrogen atoms in the glycerin molecule has been replaced by a hydroxyl group. Here the glycerol molecule has a higher molar mass \ce{$M$(Glycerin)\;=\;92.09\frac{g}{mol}} propanol molecule \ce{$M$(Propanol)\;=\; 60.10\frac{g}{mol}}.
In addition, the glycerol molecule can form other hydrogen bridges via additional hydroxy groups. Here, too, the following applies in general: the higher the number of hydroxy groups, the higher the boiling and melting point.
Flammability
Methanol Ethanol And N-propanol Are Three Common Alcohols / The flammability of alcohols depends on their boiling temperature. The lower the boiling temperature, the more steam is produced and the higher the flammability. Similar to alkanes, alcohols, whose molecules have a long organic chain, begin to produce soot when burned, since combustion is not fully realized. Therefore, the trends in the flammability of alcohols can be compared with the trends in the flammability of alkanes.
Resolution
Methanol Ethanol And N-propanol Are Three Common Alcohols: How well do various alkanols dissolve in water and how well do they dissolve in octane? water ahydrophileshydrophilic
Synonyms: hydrophilic, hydrophilic water-loving solvent and octane is a lipophilic solvent. Also in the case of solubility, we will first consider the effect of chain length and then the effect of the number of hydroxy groups.
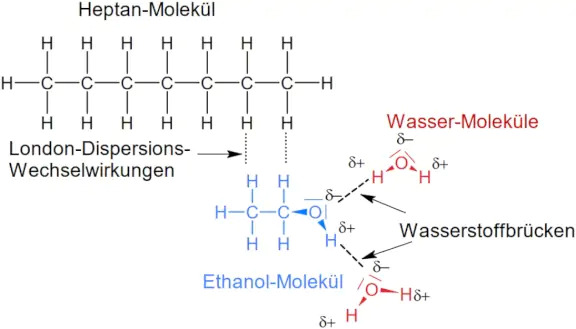
When a substance is dissolved in another substance, it means that at the particle level, attractive forces exist between molecules of different substances. For a substance to dissolve easily in water, the particles of the substance must have polar properties. In the case of alcohols, the hydroxy groups of alcohol molecules have a polar electron pair bond, so the oxygen atom has a partial negative charge and the hydrogen atom a partial positive charge (Fig. 5).
Methanol Ethanol And N-propanol Are Three Common Alcohols: Hydrogen bridges can therefore form between water molecules and the hydroxy group. However, the alcohol molecule also has an organic residue such as an alkyl chain. The CH bonds and CC bonds of the alkyl chain are non-polar, so the alkyl chain can only form London dispersion interactions. For an alcohol to dissolve well in a polar solvent such as water, the non-polar residue of the alcohol molecule must not be too large because this non-polar residue cannot form stronger interactions with water molecules.
In molecules with a very small alkyl group, such as methanol molecules and ethanol molecules, the proportion of the polar hydroxy group that can form hydrogen bonds to water molecules predominates. Therefore, methanol and ethanol are very soluble in water (hydrophilic), while octanol is very slightly soluble in water (hydrophobic). In general, therefore: In molecules with a very small alkyl group, such as methanol molecules and ethanol molecules, the proportion of the polar hydroxy group that can form hydrogen bonds to water molecules predominates.
Therefore, methanol and ethanol are very soluble in water (hydrophilic), while octanol is very slightly soluble in water (hydrophobic). In general, therefore: In molecules with a very small alkyl group, such as methanol molecules and ethanol molecules, the proportion of the polar hydroxy group that can form hydrogen bonds to water molecules predominates. Therefore, methanol and ethanol are very soluble in water (hydrophilic), while octanol is very slightly soluble in water (hydrophobic). In general, therefore: The longer the organic residue of an alcohol molecule, the less soluble the alcohol is in hydrophilic solvents such as water. / Methanol Ethanol And N-propanol Are Three Common Alcohols.
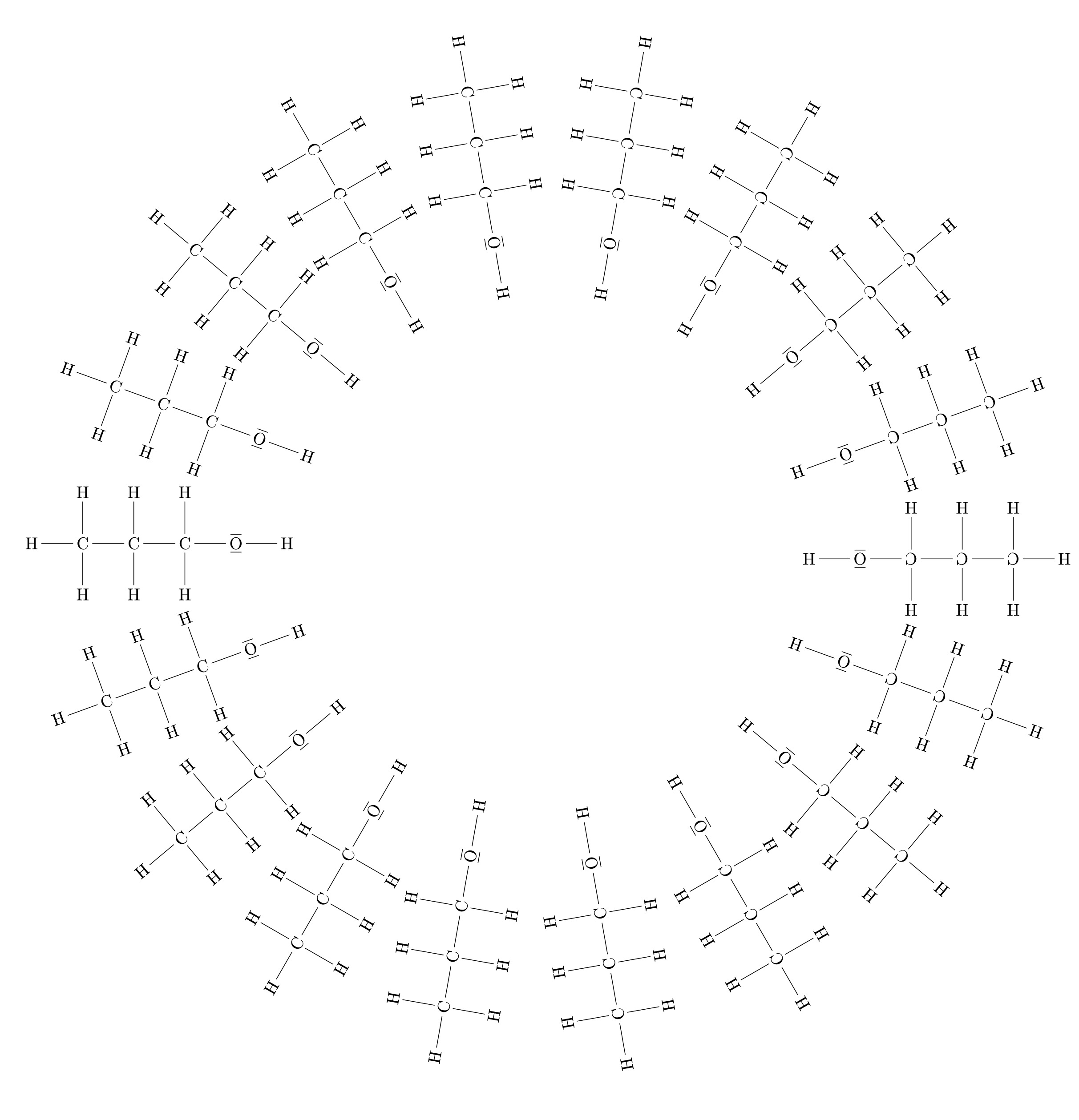
Conversely, the larger the organic residue of an alcohol molecule, the better the alcohol’s solubility in octane will certainly be. At the same time, short-chain alcohol molecules such as methanol molecules and ethanol molecules can interact with octane molecules. It is well soluble in ethanol and octane, subject to E methanol. Substances that are both hydrophilic and hydrophilic are called So how can a substance be hydrophilic and lipophilic at the same time?
Methanol Ethanol And N-propanol Are Three Common Alcohols – This is because alcohol molecules arrange themselves in a special way in a lipophilic solvent. The hydroxy groups of many alcohol molecules attract each other, so the alcohol molecules arrange themselves in a spherical fashion. The hydroxy groups of the molecules are inside and the alkyl groups are outside. These “balls of molecules” therefore behave externally as nonpolar molecules and can interact with nonpolar octanol molecules.
Let us now turn to the factor affecting the number of hydroxy groups. Here again let’s compare propanol with propane-1,2,3-triol (glycerol). Propanol is readily soluble in organic solvents, while propane-1,2,3-triol is insoluble in organic solvents. Just like the ethanol molecules in Figure 5, propanol molecules can align themselves spherically. (Fig. 6) Thus, only a non-polar residue remains outside.
This arrangement is impossible in the propane-1,2,3-triol molecule, because each carbon atom also contains a hydroxyl group. Additional hydroxy groups of alcohol molecules increase the solubility of alcohols in water, while hydroxy groups create a lower solubility in lipophilic solvents. Therefore, the following is generally true: As the number of hydroxy groups increases, the water solubility of the alcohol increases, while the chain length of the alcohol molecule remains the same.
Viscosity
Methanol Ethanol And N-propanol Are Three Common Alcohols: The greater the pull, the greater the friction. In the case of monohydric alcohols, the chain length of the molecules determines their attraction force. The longer the chain, the greater the surface area and the greater the van der Waals forces. So in general, the larger the organic residue of the alcohol molecule, the higher the viscosity of the alcohol.
In the case of polyhydric alcohols, the hydroxy groups of alcohol molecules provide a higher viscosity, as they can form relatively strong hydrogen bridges (Fig. 7). So in general, the more hydroxyl groups in an alcohol molecule with the same chain length, the higher the viscosity. Methanol Ethanol And N-propanol Are Three Common Alcohols topic is over.
 Number One Boats from USA. Boat Marketplace Group Network. All Boats & Yachts for Sale, Reviews, Specs, Prices, Craigslists.
Number One Boats from USA. Boat Marketplace Group Network. All Boats & Yachts for Sale, Reviews, Specs, Prices, Craigslists. 